2021.11.10
- Announcements
Drug Discovery to Reduce the Progression of Muscular Dystrophy by Targeting an Enzyme that Synthesizes Prostaglandin D2, Bioactive Fatty Acid
- Experiment at Kibo
Successful results obtained from JAXA Protein Crystal Growth Project
Background and interest of the research
Prostaglandins (PGs) are a group of special fatty acids with various physiological activities produced from arachidonic acid in biological membranes by the action of an enzyme called cyclooxygenase. Among them, prostaglandin D2 (PGD2) is actively produced in mast cells and immunocompetent cells that are involved in the development and progression of allergies and various inflammatory reactions such as bronchial asthma and atopic dermatitis. On the other hand, PGD2 is produced as a major PG in the brain and plays an important role in sleep regulation. There are two types of PGD2 synthases: hematopoietic PGD synthase (H-PGDS), which is involved in PGD2 production in allergy and inflammation, and lipocalin-type PGD synthase (L-PGDS), which is involved in PGD2 production during sleep. It is a wonder of life that the identical substance made from the same material in the body can be involved in the progression of inflammation and allergies, or induce sleep in the central nervous system, in other words, it has two completely different functions depending on where it is made and where it acts.
We have conducted functional studies on PGD2 in various pathological conditions, and our collaborative research group accidentally discovered that H-PGDS expression is enhanced in the muscles of the Duchenne muscular dystrophy patients. Duchenne muscular dystrophy is an inherited disease caused by an abnormal dystrophin gene on the X chromosome. It is a progressive intractable disease with muscle atrophy that occurs at a relatively high frequency of about one in 3,500 boys regardless of race, region, or country. Dystrophin is the backing protein of muscle cells and supports each individual cell. In Duchenne muscular dystrophy, an abnormality in this gene results in the complete absence of dystrophin protein, making the muscles extremely fragile, and the slightest load can easily cause inflammation of the muscles, which then progresses in a chain reaction.
We have demonstrated that H-PGDS is also expressed in and around inflamed muscles in mdx mice, which are model mice for Duchenne muscular dystrophy. We also demonstrated that administration of a prototype H-PGDS inhibitor (HQL-79), which was unexpectedly discovered, alleviated the pathology of myonecrosis in mdx mice (Figure 1). These results indicate that PGD2 produced by H-PGDS plays an important role in the progression of the pathogenesis of myonecrosis. In addition, the results show that by suppressing PGD2 production or controlling PGD2 signaling, Duchenne muscular dystrophy, once thought to be only treatable by gene therapy or stem cell transplantation, can be treated with drugs, or at least the progression of the disease can be greatly reduced.
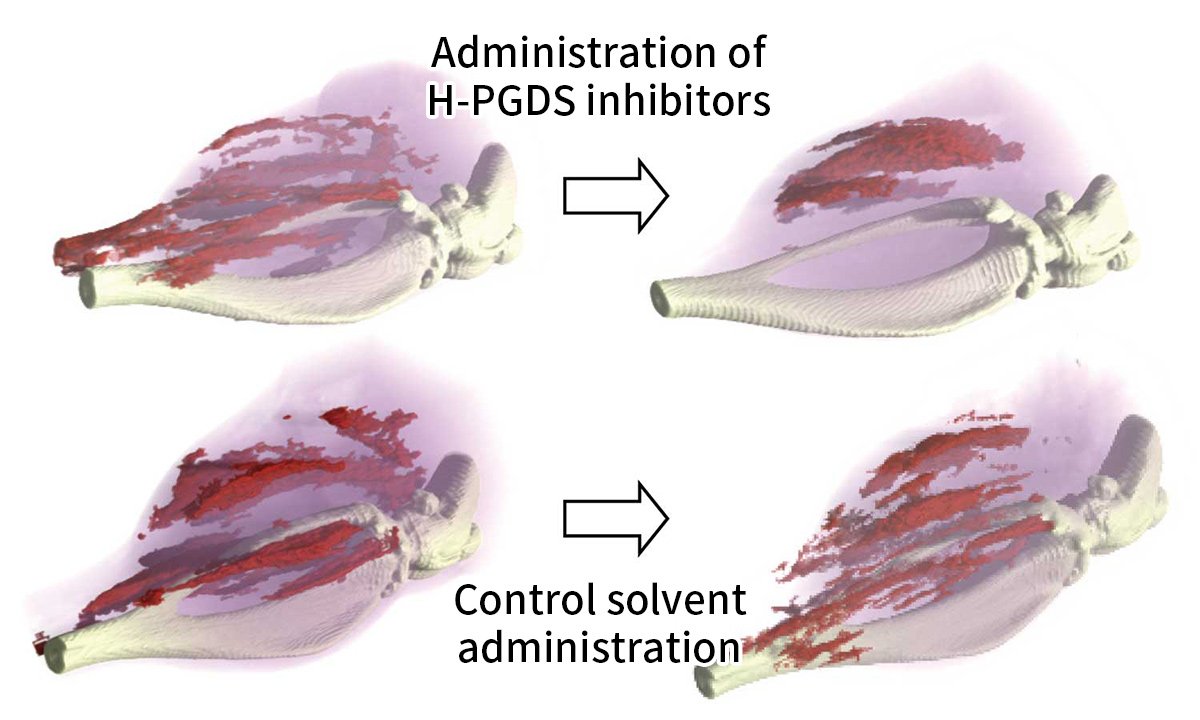
Administration of the H-PGDS inhibitor for five days reduced the number of myonecrotic areas highlighted in red. On the other hand, when only the solvent was administered, new muscle necrosis was detected.
Therefore, we set out to develop a more selective and potent H-PGDS inhibitor with fewer side effects. In order to discover drugs efficiently, it is necessary to know the precise microstructure of the catalyst site of H-PGDS. The relationship between an inhibitor and the catalytic site of an enzyme (H-PGDS here) is often compared to that of a key and a keyhole. In the past, the best drug was selected by firstly testing thousands of existing compounds randomly, selecting those with strong inhibitory activity, and then chemically modifying them. In other words, we put many keys into the keyhole to see if they would turn.
X-ray crystallography is useful to determine the detailed three-dimensional structure of H-PGDS proteins. If you know the shape of the microstructure (keyhole), you can rationally design an inhibitor molecule (key). In addition, thousands of compounds from chemical library can be screened on a computer based on the crystal structure of H-PGDS, and the optimal drug can be selected extremely efficiently.
We are working on crystallization on the International Space Station aiming to produce high-quality crystals of the protein.
Results and contribution points of space experiments
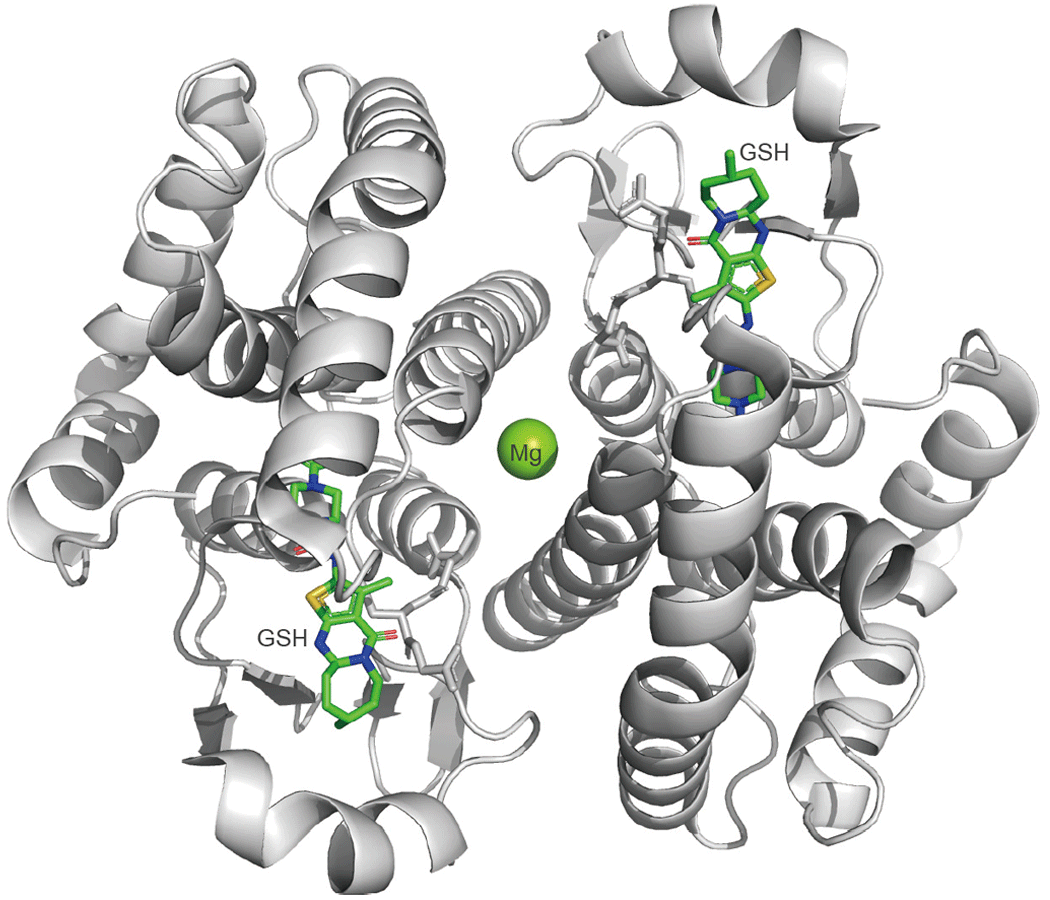
H-PGDS belongs to the gene family of σ-class glutathione S-transferases (GSTs) and, like other GSTs, forms a homodimer in vivo with a molecular weight of about 52 kDa (as a dimer form). Mg2+ is coordinated to its center, and the enzymatic reaction that isomerizes PGH2 to PGD2 absolutely requires the coenzyme GSH (Figure 2). The PGD2 synthesized by H-PGDS exacerbates the myopathy and inflammatory response of Duchenne muscular dystrophy. Therefore, in order to design a potent and selective H-PGDS inhibitor molecule, we participated in High-Quality Protein Crystal Growth Experiment on “Kibo”. We tried to crystallize a complex of the prototype inhibitor (IC50 ≑ 5 µM) and H-PGDS, and obtained crystals with a resolution of about 1.4 Å. Based on the structure determined by the experiment, selective and potent inhibitors (IC50 ≑ 10 nM) were theoretically designed and chemically synthesized. Among those novel inhibitors, one inhibitor whose efficacy and safety have been confirmed in pre-clinical studies by a Japanese pharmaceutical company, has now been confirmed to be safe in humans and are undergoing Phase III clinical trials for Duchenne muscular dystrophy from January 2021. Further experiments in space and the crystallization of a complex of the novel inhibitor and H-PGDS resulted in the formation of high-quality single crystals with an extremely high resolution (0.95 Å) in space, compared to clustered crystals on the ground. As a result, not only were the detailed three-dimensional structures of the amino acids, GSH, and inhibitors that make up the catalytic site clarified, but also the water molecules that are important for the binding of inhibitors and GSH and for maintaining the three-dimensional structure of the catalytic site were identified. This result provided useful information for molecular design and screening of new inhibitors with different basic skeletons.

Future research
Duchenne muscular dystrophy is an intractable disease caused by dystrophin gene mutations and requires long-term medication. It is also known that the progression of the disease varies from patient to patient, despite the fact that the causative gene is the same. Therefore, it is expected that the responsiveness and metabolism to therapeutic drugs will also vary.
In addition to muscle weakness, Duchenne muscular dystrophy is also associated with respiratory impairment and myocardial damage, and studies in experimental animals have shown that H-PGDS is also involved in myocardial damage and that inhibitors are effective against myocardial damage.
In the future, we would like to continue to use the opportunity of high-quality protein crystal production experiments using "Kibo" to continue molecular design and screening of new H-PGDS inhibitors with different basic skeletons, and contribute to the development of therapeutic agents for patients with muscular dystrophy.
Researchers

ARITAKE Kosuke
Professor
Daiichi University of Pharmacy

HAMAMURA Kengo
Associate professor
Daiichi University of Pharmacy
Unless specified otherwise, rights to all images belong to ©JAXA









