2021.11.16
- Announcements
Structural Analyses of Enzymes in the Rare Sugar Production Pathway
- Experiment at Kibo
Successful results obtained from JAXA Protein Crystal Growth Project
Background of the Study
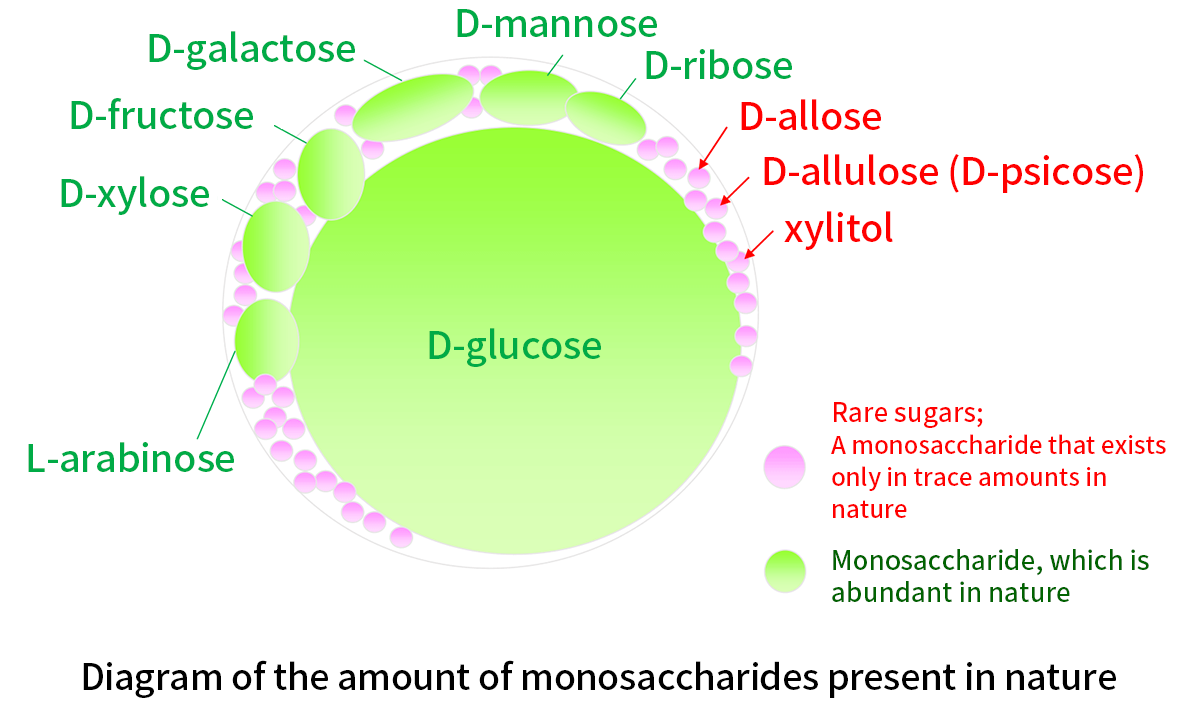
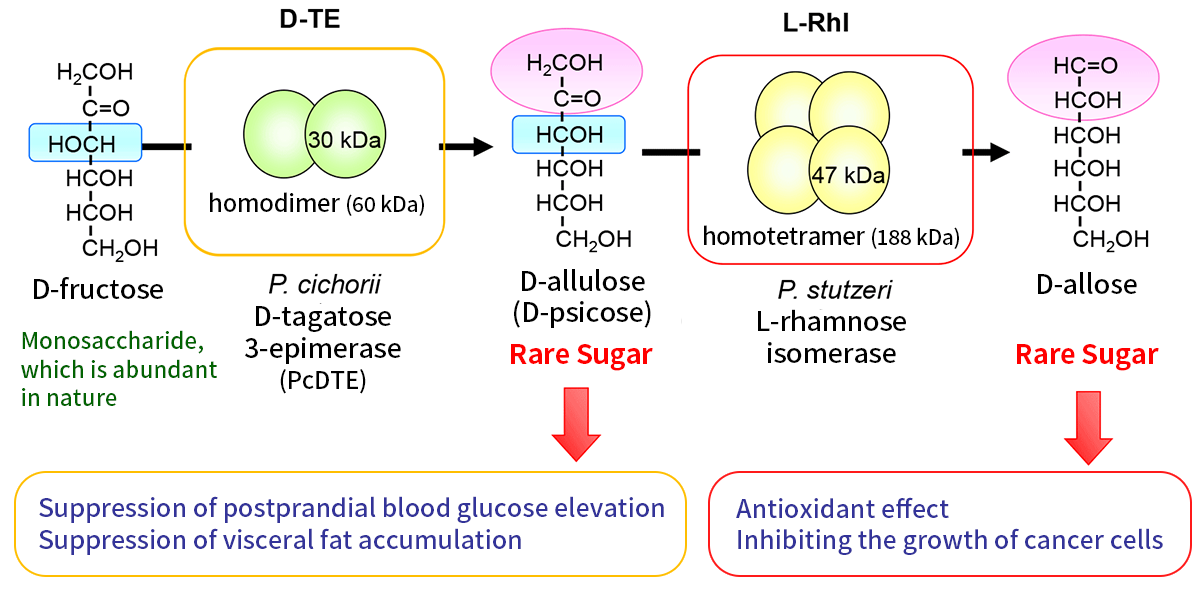
Rare sugars are defined as monosaccharides and their derivatives rarely found in a large amount in nature (Figure 1). Rare sugar research has been led by Dr. Izumori, honorary professor at Kagawa University. Due to their limited availability, rare sugars initially drew very little attention or rarely studied as a scientific project. Professor Izumori and his group discovered the first enzyme producing D-allulose (D-psicose), a rare sugar made from D-fructose abundant in nature1-3). This result achieved by the group triggered a series of D-allulose studies, where D-allulose produced by the enzyme was applied. The research revealed physiological effects of D-allulose, including inhibitory effects on blood glucose level and on accumulation of endovascular fat. The second enzyme, which enables to produce another rare sugar, D-allose from D-allulose, was discovered, D-allose was reported to show an antioxidative effect and even a growth inhibition effect on cancer cells (Figure 2). The amount of rare sugar in nature is extremely low, on the other hand there are many kinds of rare sugars. Professor Izumori designed a blueprint, "Izumoring," 4) for production of various rare sugars. Izumoring has generations and is still evolving.
In the past, D-allulose, merely an unknown rare sugar, is now approved as GRAS (Generally Recognized As Safe) by the U.S Food and Drug Administration (FDA). The effects and values of D-allulose are recognized in medicine and health care fields as well as in the food industry as a no calorie sweetener. It is also reported to be involved in disease resistance induction and growth control in plant.
https://www.kagawa-u.ac.jp/IIRSRE/en/index.html
https://www.kagawa-u.ac.jp/IIRSRE/index.html (in Japanese)
A plant called Itea produces D-allulose but it is still unknown why only Itea produces it. To promote the research on the rare sugar tree Itea, it is cultivated by a local group “Komino-Zuinaers” (Komino is a place in Kagawa prefecture, where the Rare Sugar Research Center based. Zuina means Itea in Japanese. They work at an unexpected place and grow Itea saplings of as a gift for children.) (Picture 1)
http://www.izumoring.com/index.html (in Japanese)
http://www.izumoring.com/index.html (in Japanese)


D-allulose is is not the only rare-sugar but many rare sugars are known. Although rare sugars are monosaccharides and their structures are simple structures, they are mostly unknown. By producing rare sugars, we could study how to utilize them as well as why such rare sugars continue to remain in nature.
In the rare sugar research, functional analysis and industrial application of individual rare sugars are studied. In addition, screening enzymes and developing chemical synthesis methods aiming to a large-scale production are performed. Large chunk of crystals of a rare sugar can be available thanks to such efforts. (Picture 2)
Our research group has been focused on structural analyses of rare sugar producing enzymes to reveal the catalytic mechanisms including how the enzymes capable of recognizing and producing rare sugars.
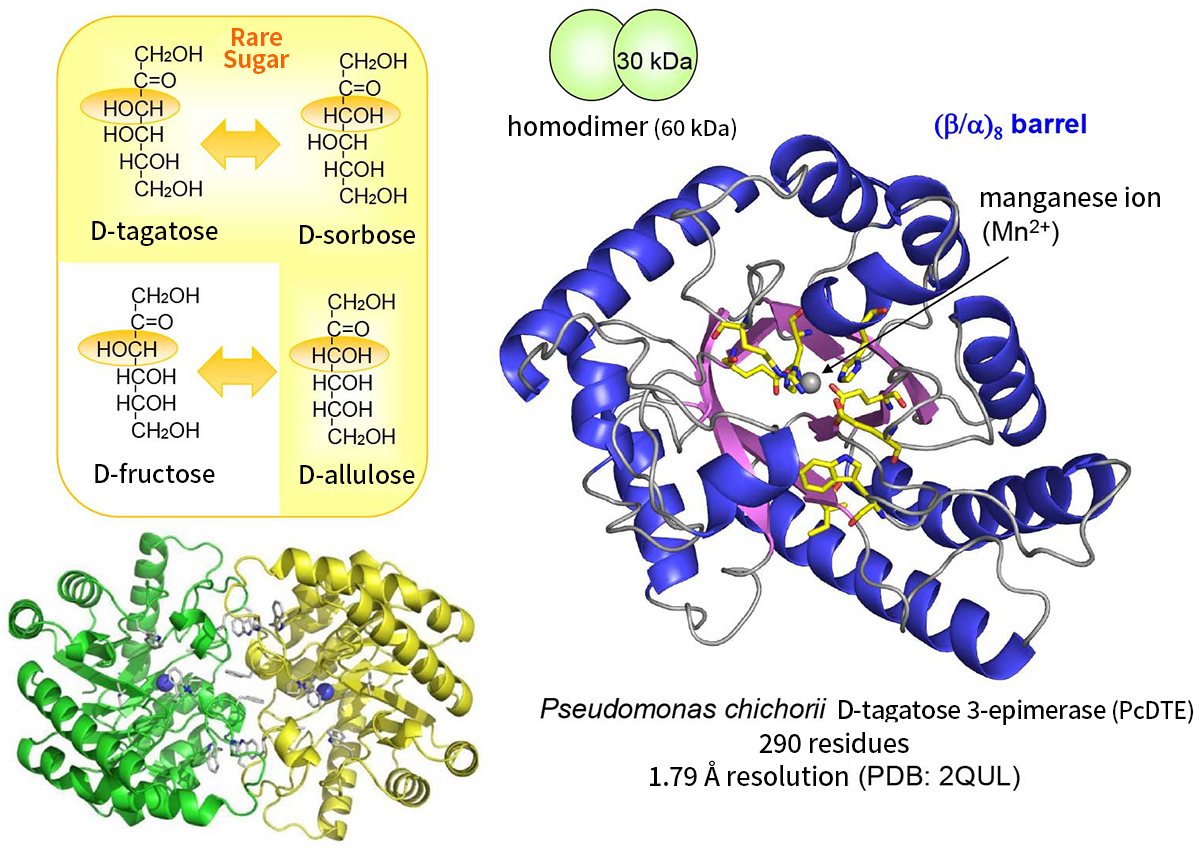
The first enzyme identified as a rare sugar producing enzyme was D-tagatose 3-epimerase (PcDTE) from Pseudomonas cichorii, which catalyzes epimerization between D-tagatose and D-sorbose. PcDTE also epimerizes 3-hydroxyl group between D-fructose and D-allulose. The enzyme can epimerize 3-hydroxyl group of ketoses but does not take a phosphorylated sugar as a substrate but reacts to 3-hydroxyl group of non-phospholylated monosaccharides. The discovery of this enzyme was the breakthrough for the large scale production of D-allulose from D-fructose.
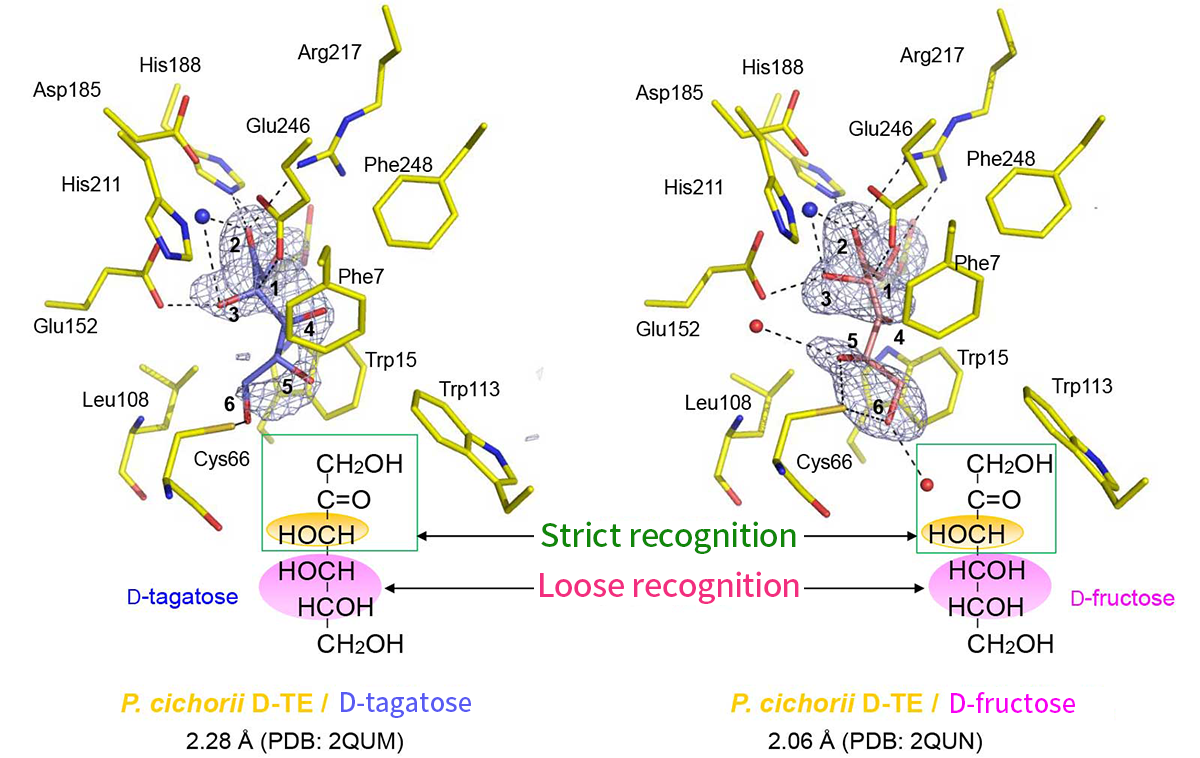
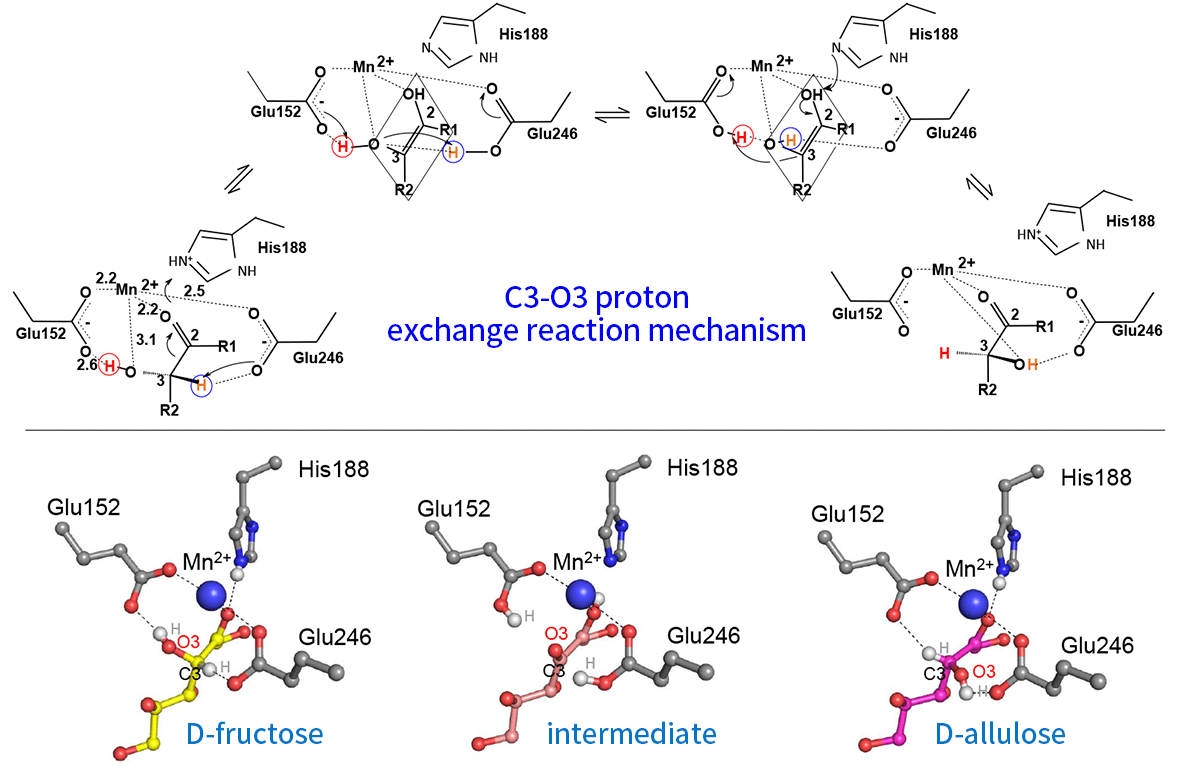
X-ray crystallographic analyses of PcDTE revealed 1- and 3-hydroxyl groups and 2-keto group of the substrates were tightly recognized in the active site, but the recognition of 4-, 5- and 6- hydroxyl groups was weak. This finding explains the fact that PcDTE recognizes D-fructose and produces D-allulose(Figures 3 and 4). Structural analysis of a PcDTE-substrate complex shown that two Glu residues (Glu152 and Glu246) worked as the catalytic residues by interacting to the both sides of the 2-keto group and 3-hydroxyl group of the substrate. Thus, we have proposed a catalytic mechanism "C3-O3 proton exchange reaction", where a proton exchange occurs between position C3 and O3 of the substrate based on the cis-enediol reaction mechanism (Figure 5) 5).
Results of Space Experiments
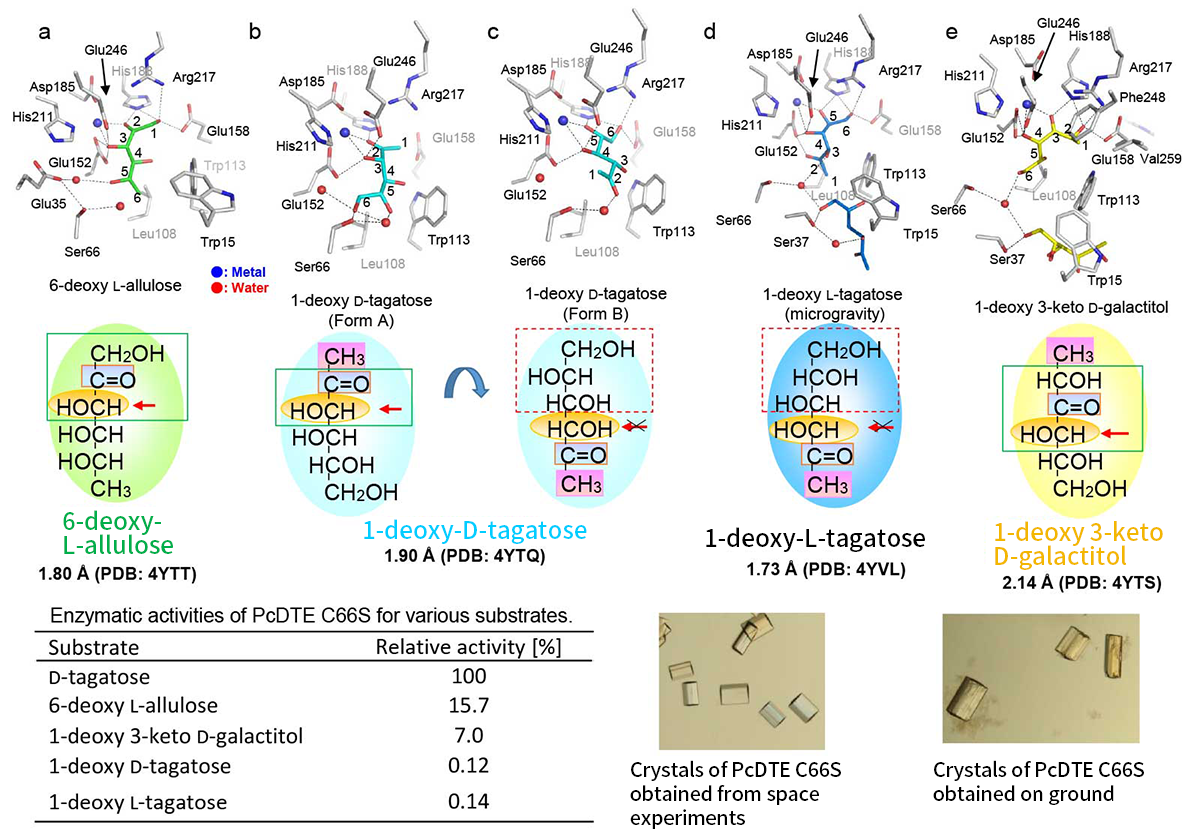
We found that PcDTE can act on 1-deoxy sugar and even 1-deoxy 3-keto D-galactitol, which has no 2-keto group, as substrates. We then conducted X-ray crystallographic analyses of complexes with various substrates to understand how these substrates are recognized by PcDTE. To obtain high quality crystals for improving the resolution, we have participated JAXA’s “High-Quality Protein Crystal Growth Project”. Excellent crystals were grown in microgravity. Using crystals obtained from both on the ground and in space, we have revealed the interesting substrate recognition mechanism of PcDTE characterized by wide substrate specificities6).
We also found that, on the active site of a mutant PcDTE Cys66Ser, which maintains an enzymic activity similar to the wild-type. In some conditions, 2-keto and 3-hydroxyl groups of 1-deoxy sugar were recognized and was catalyzed as a substrate, however, in other cases, 6-, 5- and 4-hydroxyl groups were bound as an inhibitor-binding mode with no further reaction. This can explain the fact that PcDTE shows activity to 1-deoxy sugars but its overall activity is low. With 1-deoxy 3-keto D-galactitol, 2- and 4-hydroxyl groups (one shift at substrate-binding site) and 3-keto groups are recognized and form a more stable substrate than 1-deoxy sugars. These findings show why PcDTE has higher enzymic activity to this substrate than 1-deoxy sugars (Figure 6).
Future research activities
Enzymes involved in rare sugar production are characterized by their wide substrate specificities. One of such enzymes PcDTE shows interesting characters. By solving structures of various rare sugar producing enzymes with unique characters, we are going to identify the properties and catalytic mechanisms of enzymes, and to contribute to improved rare sugar production through molecular design.
References
- Izumori, K., The Secret Story of Rare Sugars, 2013.
- Khan, A. R., Takahata, S., Okaya, H., Tsumura, T. & Izumori, K., J. Ferment. Bioeng., 74, 149 (1992).
- Izumori, K., Khan, A. R., Okaya, H. & Tsumura, T., Biosci. Biotechnol. Biochem., 57, 1037 (1993).
- Izumori, K., Naturwissenschaften, 89, 120 (2002).
- Yoshida, H., Yamada, M., Nishitani, T., Takada, G., Izumori, K. & Kamitori, S., J. Mol. Biol., 374, 443 (2007).
- Yoshida, H., Yoshihara, A., Ishii, T., Izumori, K. & Kamitori, S., Appl. Microbiol. Biotechnol., 100, 10403 (2016).
Researchers

YOSHIDA Hiromi
Associate Professor
Kagawa University

KAMITORI Shigehiro
Director of LSRC & Professor
Kagawa University

YOSHIHARA Akihide
Assistant Director & Associate Professor
Kagawa University

IZUMORI Ken
Research Adviser & Professor Emeritus
Kagawa University
Unless specified otherwise, rights to all images belong to ©JAXA









