- completed
[Bisphosphonates]
Bisphosphonates as a Countermeasure to Spaceflight-Induced Bone Loss
- Human Research
ISS Science for Everyone
SCIENCE OBJECTIVES FOR EVERYONE
The purpose of the Bisphosphonates as a Countermeasure to Space Flight Induced Bone Loss study is to determine whether an antiresorptive agent, in conjunction with the routine in-flight exercise program, protects International Space Station (ISS) crew members from the regional decreases in bone mineral density documented on previous ISS missions.
SCIENCE RESULTS FOR EVERYONE
ISS crew members exercise regularly to help reduce bone loss but still lose signifcant bone mass over a long mission. A weekly pill may help. The Bisphosphonates as a Countermeasure to Space Flight Induced Bone Loss study tests biphosphonates, bone antiresorptive agents used to increase bone strength, in conjunction with routine in-flight exercise during long-term spaceflight. Bone measurements and comparisons of early model resistance exercise device or the advanced resistance exercise device (ARED) show that ARED and bisphosphonate together improve essentially all measures of bone physiology during spaceflight. Scientists conlude that exercise plus an antiresoptive drug may help prevent bone loss on long missions.
Experiment Description
RESEARCH OVERVIEW
- The potential for loss of bone mass is one of the most important medical concerns for long-duration manned space flight with regional losses of 1 to 2 percent per month in spite of the fact that crew members exercise while in space. The resultant hypercalciuria (abnormally high calcium levels in urine) increases the risk of renal stone formation.
- Bisphosphonates are a group of antiresorptive agents that block the breakdown of bone and are used to treat osteoporosis and other disorders related to bone turnover.
- This study tests the effectiveness of alendronate, taken as a pill once per week before and during space flight.
- If shown to be an effective countermeasure to space flight-induced bone loss, bisphosphonates or other antiresorptive agents could help prevent several bone-related problems for crew members on the International Space Station (ISS) and on future long-duration missions. These problems include loss of bone mineral mass and strength and the possibility of developing renal stones during or after space flight.
DESCRIPTION
The purpose of this investigation is to determine whether bisphosphonates, in conjunction with the routine in-flight exercise program, protects International Space Station (ISS) crew members from the regional decreases in bone mineral density documented on previous ISS flights. Two dosing regimens were originally planned, an oral dose of 70 mg of alendronate taken weekly starting 3 weeks prior to flight and then throughout the flight and an intravenous (I.V.) dose of zoledronic acid, 4 mg, administered just once approximately 45 days before flight. However, only alendronate administration was actually tested. Control subjects, who participate in all pre-, in-, and post-flight tests, but do not ingest an antiresorptive agent, were added to control for the effects of improved exercise equipment aboard the ISS.
An interim review of the data for this study (04-E255/SMO 021) indicated that it was necessary to add a new control group whose results could be compared to those of crewmembers who have taken bisphosphonates. The original historical control group (14 crew members from early ISS flights) exercised using the Interim Resistive Exercise Device (IRED), while the bisphosphonate subject group (7 subjects, now complete) exercised on the newer Advanced Resistive Exercise Device (ARED). The new control group will help distinguish the relative effects of bisphosphonates versus the confounder of ARED. The control subjects will participate in essentially the same data collection protocol as the bisphosphonate group, but will not take the oral or i.v. bisphosphonate.
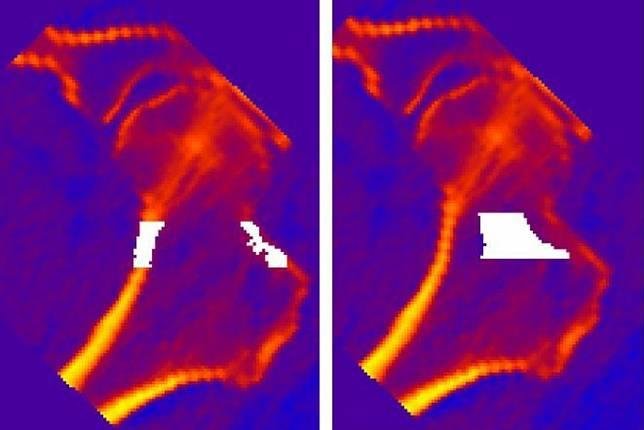
The primary measurement objective of this study is to obtain preflight and postflight Quantitative Computed Tomography (QCT) scans of the hip. The QCT scans provide volumetric bone density information of both cortical and trabecular (spongy) bone regions of the hip. This study aims to show that bisphosphonates significantly reduces bone mineral density loss and the increased risk of renal stone formation documented previously on untreated ISS crew members.
Secondary measurement objectives include: preflight and postflight Dual-energy X-Ray Absorptiometry (DXA) scans of the whole body, spine, hip, and heel; preflight and postflight scans of the tibia using peripheral Quantitative Computed Tomography (pQCT); preflight and postflight abdominal/retroperitoneal ultrasound scans; preflight and postflight blood draws to measure serum markers of bone metabolism, and preflight, in flight, and postflight urine collections to measure urinary markers of bone metabolism. Urine measurements are also used to look at the risk for developing renal stones before, during and after flight.
Media Gallery
Applications
SPACE APPLICATIONS
The purpose of this investigation is to determine whether antiresorptive bisphosphonates, in conjunction with the routine in-flight exercise program, will protect ISS crew members from the regional decreases in bone mineral density and bone strength documented on previous ISS flights. If shown to be an effective countermeasure to space flight induced bone loss, bisphosphonates could prevent or ameliorate several potential bone-related problems identified in NASA's Critical Path Roadmap. If bisphosphonates improve the efficiency of in-flight exercise to maintain bone mass, then more crew time could be made available for other purposes.
EARTH APPLICATIONS
The benefits of this research are primarily for space travelers. Knowledge gained from this investigation may generate useful information applicable to patients on Earth with accelerated bone loss due to disuse (e.g., spinal cord injury patients or those with prolonged immobilization).
Operations
OPERATIONAL REQUIREMENTS AND PROTOCOLS
This experiment requires the participation of long-duration crew members taking the drug compared with 10 control subjects. All subjects will complete DXA scans (Launch minus 45 days (L-45), Return plus 5 days (R+5), R+180, [not required for controls], and R+360), pQCT scans (L-45, R+5, R+180, [not required for controls], and R+360), high resolution QCT scans (L-45, R+5, R+360), ultrasound scans (L-180, R+30), 24-hr urine collections (L-45, R+0, early inflight, midflight, late inflight, R+30, R+360), and blood draws (L-45, R+0, R+30, and R+360). Alendronate subjects will complete an Alendronate Tolerance Test on L-180, and they will take Alendronate on L-17, L-10, and L-3.
While in flight, Alendronate subjects ingest one pill weekly. All subjects conduct three urine collection sessions (4 weeks, 12 weeks, and 24 weeks). Crew members also take a daily Vitamin D supplement for the duration of the mission.
Publications
PRINCIPAL INVESTIGATOR(S)
MATSUMOTO Toshio [Tokushima University] Jean Sibonga [NASA]
Unless specified otherwise, rights to all images belong to ©JAXA



