
02

Recently, the remarkable progress of biotechnologies has generated a new class of medicine, biopharmaceuticals such as antibodies. Biopharmaceuticals with large molecular size make multipoint molecular recognitions against the targeted disease-related proteins to show specific binding ability and thereby induce high therapeutic efficacy with no side effects. On the other hand, the large molecular size causes several limitations, a lack of cell membrane permeability, immunogenicity, high cost to manufacture and so on.
To overcome the limitations, we have designed molecular-targeting peptides with a helix-loop-helix (HLH) structure as a next-generation biopharmaceutical. Despite being 50 times smaller than antibodies, the HLH peptide shows antibody-like functions, high affinity and high specificity for the targeted proteins.
Using directed evolution in a phage-displayed library of HLH peptides, we succeeded to generate a binding peptide to human vascular endothelial growth factor (VEGF). VEGF, a cytokine inducing pathological angiogenesis, is the therapeutic target for macular degeneration and colon cancer. The HLH peptide strongly binds to VEGF and does not bind to the other proteins. The high binding specificity indicates that the HLH peptide has a very high potential as a next-generation biological drug.
To lead the HLH peptide to a therapeutic, we needed a high-resolution 3D structure that enables us to explain the binding mode between the HLH peptide and VEGF. Therefore, we applied for participation in the high-quality protein crystal growth experiment. In the 12th space experiment, we obtained a protein crystal and defined a 3D structure of the HLH peptide-VEGF complex at 1.46Å resolution.
Using 3D structural information, we attempted to design a conjugate of the HLH peptide with a peptide inhibitor (v108) to VEGF-VEGFR interaction. In the design, the linking sites on each peptide and the linker length was precisely calculated from the high-resolution 3D structure. Finally, we successfully generated a very strong VEGF inhibitor. Although peptide v108 has a very poor inhibition activity, the HLH peptide-v108 conjugate strongly inhibits VEGF-VEGFR interaction with the synergistic effect by bivalent binders.
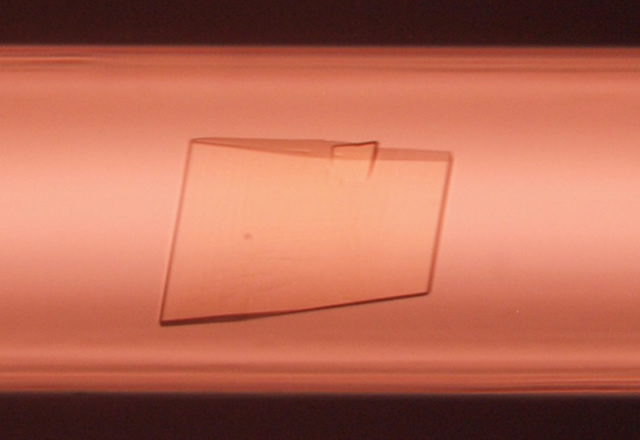
Fig. 1: A protein crystal of the complex of HLH peptide and VEGF
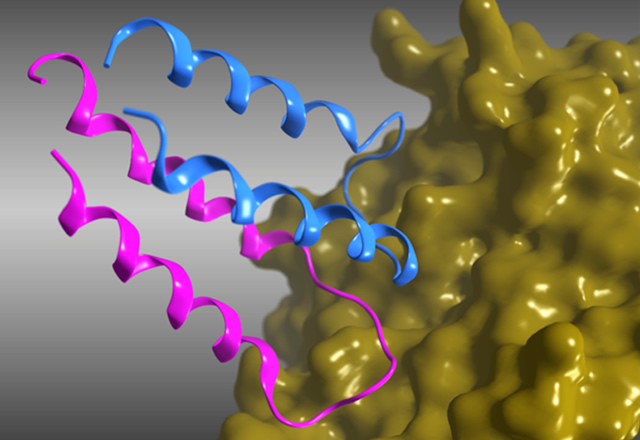
Fig. 2: High-resolution crystal structure of the HLH peptide-VEGF complex. HLH peptides were presented by blue and violet ribbon, and VEGF were presented by yellow surface
Patent application based on data obtained during space experiments
Published patent application 2019-196326: VEGF binding inhibitorIn the near future, we have a plan for animal experiments of the HLH peptide, which we designed in this study. The data obtained from the space experiments were very beneficial and allowed us to accelerate our drug discovery research. We have developed HLH peptides for various target proteins. We will continue to conduct drug discovery research on such peptides, utilizing the opportunity of space experiments.

Professor Ikuo Fujii

Assistant Professor Masataka Michigami