
02

Chitin is the main constituent of the outer skeleton (shell) of crustacea (lobster and crab) and insects. It is also contained in the cellular barrier of fungus and champignon and is a persistent macromolecular polysaccharide that works as a constituent of strength. It has a long structure composed of hundreds or thousands of N-acetylglucosamine (a kind of amino sugar) linked with each other like a chain. The category of arthropod to which crustacea and insects belong is the largest animal group on the Earth. Next to cellulose, constituent of the cellular barrier of vegetable cell, chitin is the richest bioresource. The amount of chitin synthesized on Earth is estimated to be one hundred billion tons per year.
In addition to the richness as a resource, chitin has an excellent biological compatibility. In fact, it has been used for manufacturing medical supplies such as artificial skin and surgical suture. However, this persistent and insoluble polysaccharide has not economically been utilized because of the difficulty in handling and processing. In the meantime, it was proved that N-acetylglucosamine, its constituent sugar, has remedial effect on knee osteoarthritis and esthetical effect on skin. The food industry has started to use chitin as an ingredient and the demand for an N-acetylglucosamine-containing functional food (brand name: "Glucosamine") against aging has worldwide increased. Thus, chitin has rather become known to the public as an ingredient of Glucosamine. As raw materials, the industry mainly uses shells of lobster and crab disposed of in large amounts in seafood processing for producing frozen foods and canned seafood.
Hydrolytic degradation of chitin using a strong acid (e.g. concentrated hydrochloric acid) might be applied to the production of glucosamine because of its advantage of reducing the producing costs. This method however generates deacetylation during degradation and makes the material chemically unstable. Besides, deacetylated glucosamine has a unique uncomfortable taste in contrast to N-acetylglucosamine which has a refreshing sweet taste and is not suitable for food production. Thus, though it has been known that hydrolytic degradation of chitin using an enzyme is suited for producing N-acetylglucosamine, a powerful degradative enzyme capable of degrading persistent and insoluble polysaccharide has not been known.
Thus, we thought of placing a trap composed of a large amount of chitin in the soil of a farmland in Fukui Prefecture to capture microbes having strong chitin degrading capability. The trap successfully worked and many chitin degrading microbes were captured. We chose a type of microbes which had the strongest degrading ability and named it Paenibacillus sp. FPU-7 strain. Microbes of the genus Paenibacillus widely exist on the Earth. We discovered that the FPU-7 strain is capable of directly degrading insoluble chitin of crab shell and succeeded for the first time in the world in developing a technique for fermentation and production of N-acetylglucosamine (Fig. 1).

Fig. 1: Degradation of Crab-Shell Chitin by Paenibacillus sp. Strain FPU -7
The chitin-degrading mechanism of the FPU-7 strain is very attractive for industries and scientists. We then tried to identify the chitin-degrading enzymes. A large quantity of chitin-degrading enzymes (chitinase) had been secreted in the culture supernatant of the FPU-7 strain. These enzymes were subjected to genomic analyses and twelve types of chitinase gene (ChiA to ChiW) were identified. Then we discovered that ChiW plays a principal role in degrading insoluble chitin. The enzyme ChiW was proved to have the number of amino acid residues over one thousand and be huge in size for microbe-derived enzyme. In general, chitinase is an enzyme that is secreted from a cell. ChiW is a very characteristic chitinase because its molecules are combined with each other on the cell surface and one molecule contains two catalytic domains for chitin degradation. To identify a more precise molecular structure of ChiW and improve its functions, we have decided to attempt its crystallization in space experiments.
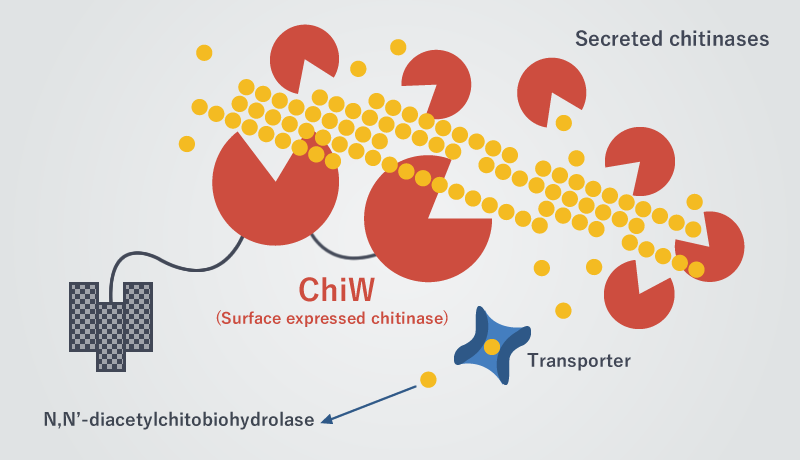
Fig. 2: Twelve types of chitinase of FPU-7 strain degrade chitin
ChiW is a huge protein that has a molecular weight of 150,000 and consists of six domains: S-layer binding domain (SLH), CBM54 saccharide binding module, immunoglobulin-like domain-1, GH18 catalytic domain-1, immunoglobulin-like domain-2 and GH18 catalytic domain-2. We have prepared two types of protein samples for crystallization: protein deprived of SLH (ChiW-SLHD; molecular weight of 130,000) and protein deprived of SLH and CBM54 (ChiW-CD; molecular weight of 90,000). We have searched crystallization conditions and found that both types of protein samples had been crystallized in the presence of ammonium phosphate and citrate buffer solution (pH: 5.5). Crystals of ChiW-CD diffracted up to 2.0 Å and those of ChiW-SLHD (Fig. 3) diffracted up to 2.9 Å.
Hoping to improve the crystal quality, we applied for the Kibo "high-quality protein crystal growth" project. At the PCG-5 experiment, the resolution of the crystals of ChiW-SLHD was improved up to 2.6 Å as expected. It was proved that ChiW-SLHD nucleation is enhanced in space. This was a useful finding for subsequent attempts to improve the crystal quality. Even with inactive variant of ChiW-CD, crystals were generated. The steric structure of a complex with chitin oligosaccharide (pentasaccharide) was determined with 2.6 Å resolution.
We continued the space experiment on PCG-6 to PCG-8. During these periods, we shifted the protein sample purification tag from the C-terminal to the N-terminal, and also modified the sequence of the N-terminal. These measures contributed to improving the quality of crystals. Further trials and errors for crystallization conditions and subsequent space experiments allowed the crystals of ChiW-SLHD to attain 2.1 Å resolution (Fig. 4).

Fig. 3: Crystals of ChiW-SLHD before the collaboration with JAXA

Fig. 4: Crystals of ChiW-SLHD grown under microgravity
The 3D structure of ChiW-SLHD was determined by molecular replacement using ChiW-CD as template (Fig. 5). The determined structure of ChiW-SLHD was utilized to prove that this enzyme has a complex multidomain structure consisting of five (or six if SLH is included) domains including two GH18 catalytic domains. Furthermore, the glycine-rich flexible loop structure of 35 Å in length which links a ?-helix structure with the catalytic domains was clearly identified. The newly identified molecule structure suggests that ChiW smartly utilizes the characteristic domains to effectively degrade chitin of hard cellular barrier (Itoh et al., PLos One. 11, e0167310, 2016).
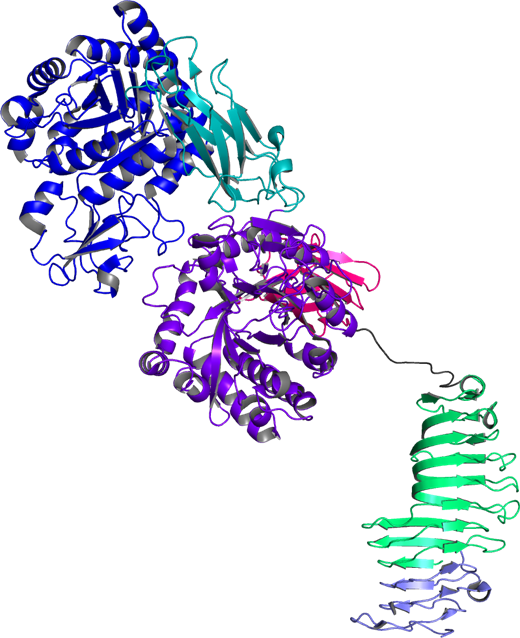
Fig. 5: Structure of ChiW-SLHD crystal determined with 2.1 Å resolution
We have successfully developed the fermentation and production techniques for N-acetylglucosamine, a constituent sugar (monosaccharide) of chitin. Our next scientific concern is about a production method for chitin oligosaccharides. Researchers' attention is being attracted by the elicitor activities of chitin oligosaccharides which activate the innate immune system of animals and plants and evoke their defense mechanism. In fact, "antitumor activity for humans and animals" and "plant's resistance improving activity" have been identified in chitin oligosaccharides.
Recently, scientists, especially those from European countries, are showing a strong interest in natural substances capable of enhancing/benefitting nutrient uptake, nutrient efficiency, tolerance to abiotic stress, and crop quality of plants. Such substances are referred to as biostimulants. Biostimulants such as chitin oligosaccharides are expected to improve the disease resistance and environmental stress resistance, yields, qualities and shelf lives of agricultural crops as well as the use efficiency of fertilizers.
Chitin oligosaccharides however have a low production efficiency in enzymolysis and acidic cleavage. High-efficiency production methods are required. The chitinase gene ChiW which we discovered has hydrolysis activity and chitin synthesis activity through transglycosylation. Making use of this activity, we hope we will be able to develop a technique for producing chitin oligosaccharides.
