
03

Technical Information01.
Crystallization Containers and Methods
Technical Information02.
Protein Characterization as Quality Control
The Gel-tube crystallization method is illustrated in this page.
A step-by-step procedure is available with a movie.
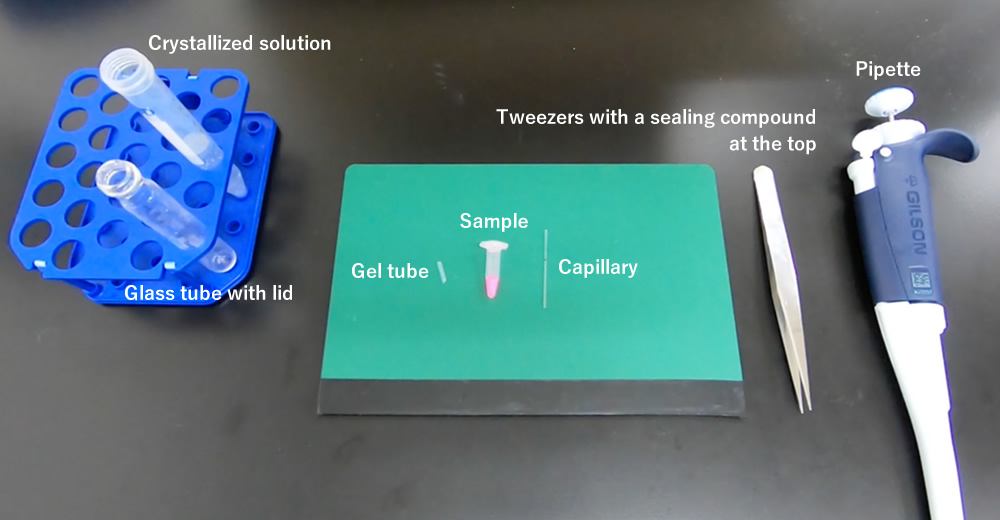
A kit including all items necessary for the gel tube method can be purchased from here (Japanese).
Confocal Science Inc.The following is an example of the products if you purchase separately.
EM Meister RingcapsR 5 μL (inner diameter of 0.3 mm, 2-454-01) or 10 μL (inner diameter of 0.5 mm, 2-454-02) (manufactured by AS ONE Corporation) Cut with capillary cutter when necessary.
Equivalent product available
47 mm-cut capillary purchasable at Confocal Science Inc.
Gel tube (MB2000-CRT202, manufactured by Confocal Science, Inc.)
Product PageGraduated test tube (screw cap) 10 ml, round bottom (6-768-03, manufactured by Maruemu Corporation).
Substitutable with one that can house precipitant and capillary.
Hemato-Seal capillary tube sealant (02-678,manufactured by Fisher Scientific, a brand of Thermo Fisher Scientific Inc.)
Product PageSILASCON® medical tube (inner diameter of 1.0 mm; outer diameter of 2.0 mm) (100-1N)
Product PageAgarose S (312-01193, manufactured by Nippon Gene Co., Ltd.)
Substitutable with one at electrophoresis level or higher
Easier to handle without lock base to connect with SILASCON® tube
Step01.
The solution should be completely dissolved by using such equipment as a microwave oven. Use pure water for solvent.
Step02.
For easy handling, you can cut a long tube into about 30 cm long in advance.
Step03.
Step04.
Step05.
Step06.
Provide preservative treatment, such as adding azide, when necessary.
Note: Do-it-yourself gel tube may not provide performance equivalent to that of a commercial product.
A few days before to immediately before the operation
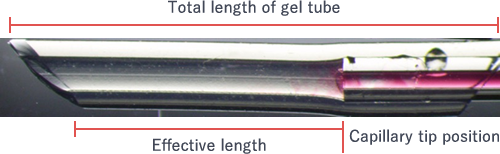
The standard cut length is 15 mm. Adjust the length properly to facilitate subsequent operation because the standard effective length (*) of a gel tube after connecting with a capillary is 9 mm.
(*) The length from the capillary edge in the gel tube to the surface accessing the external solution when the capillary is connected with a gel tube (see the figure).
The standard length is 47 mm.
Categorized into the following three basic patterns. Changeable accordingly.
Immediately before operation
The standard method is mixing the protein solution and crystallized solution at a 1:1 ratio. For seeding, the 1:1 ratio mixture is recommended to avoid the dissolving of seeds, enabling crystals to be obtained earlier than when solely using protein solution as the solution for filling the capillary.
Step01.
The standard solution amount is about 1 ml (when using the glass tube introduced in the article description). Changeable accordingly.
Step02.
Sample with an inner diameter up to 0.5 mm can be filled by applying capillary action. A capillary is filled with sample by absorbing after connecting with an article, such as silicon tube when necessary.
(Sample consumed)
When the sample length is 40 mm, about 3 μL of sample for inner diameter of 0.3 mm and 8 μL for inner diameter of 0.5 mm are consumed.
Step03.
A small amount of compound on the upper tip of tweezers helps operation. More helpful when softened by kneading immediately before use.
The procedure goes well by using the compound for the first few times just like pressing. Filling About 3-mm compound works sufficiently.
(Common trouble: Case 1)
When the compound is filled into the capillary, gas phase sometimes occurs at the lower edge because sample moves to the upper edge. Leaving the gas phase does not allow crystallization to progress. Some millimeters of gas phase at the lower edge of the capillary can be pushed out by repeatedly filling the compound. However, when more than some millimeters of gas phase occur, the sample should be refilled in the capillary.

Sample can be collected by pushing it out after the capillary is connected with a silicon tube and a pipette tip.
Step04.
Set a gel tube and a capillary in a mound shape, and then squeeze the capillary in the gel tube at one breath. Screwing the capillary while rotating it a little makes it easier to squeeze. About 4 mm is sufficient for the insertion length.
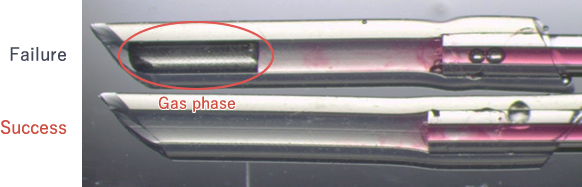
(Common trouble: Case 2)
Gas phase occurring between the lower edge of the gel tube and the external solution hampers the progress of crystallization. The operation should be repeated as the situation cannot be basically recovered.
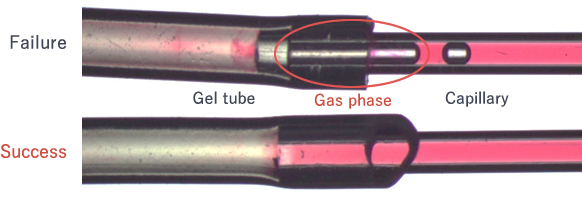
See Common trouble: Case 1 for the sample collection method
Step05.
The standard effective length of a gel tube* after connecting with a capillary is 9 mm. The tube should not be necessarily cut because the impact of the effective length on crystallization is observed to some extent, but usually below its need of awareness.
(*) The length from a capillary edge in a gel tube to a surface accessing the external solution when a capillary and a gel tube are connected.
Step06.
(Common trouble: Case 3)
Gas phase occurring between the lower edge of the gel tube and the external solution hampers the progress of crystallization. When a gas phase is confirmed, you should take out the capillary and inject the solution into it with PIPETMANR or another tool to push out the gas phase from the lower edge.
Step07.
Do not keep the glass tube laid on its side for a long period of time, which may cause the external solution to flow rapidly into the capillary and hamper the solute's concentration gradient from forming. (No effects with a counter diffusion method will be obtained.)
No problem if a glass tube is laid on its side only for a short period of time, for example, at crystal observation.
Step08.
When encapsulated in a glass tube, crystals can be fully observed through the glass. If it is difficult to observe through the container, capillary may be removed from the container and directly observed for a short period of time, during which no problems occur, including being dry.
In addition to the method above, a dialysis method where the dialysis membrane is placed between a capillary and a gel tube is also available. If interested, feel free to contact us.