Interview
04

Interview01.
Artificial Blood achieved through 20 years of passionate research
Interview02.
Getting to the bottom of human's greatest infection periodontal disease
Interview03.
True sustainable society proposed by a biomass researcher

Yasumitsu Sakamoto
Assistant professor, School of Pharmacy, Iwate Medical University
1971: Born in Kumamoto, raised in Saitama.
2002: Withdrawal from the Doctoral Program of Nagaoka University of Technology with the Completion of Course Requirements.
2002: Assistant professor; School of Nursing and Rehabilitation Sciences, Showa University, Japan.
2005: Received Ph.D. from Showa University.
2007: Lecturer; School of Nursing and Rehabilitation Sciences, Showa University.
2008: Assistant professor; School of Pharmacy, Iwate Medical University.
2016: SSH management and mentorship; Omiya Kita High School, Saitama, Japan.

Wataru Ogasawara
Professor, Department of Bioengineering, Nagaoka University of Technology
1969: Born in Iwate.
1997: Received Ph.D. from Nagaoka University of Technology.
1997: Assistant professor; Faculty of engineering, Iwate University.
1998: Postdoctoral Fellow; University of Bristol.
2008: Research Associate Professor; Top Runner Incubation Center for Academia-Industry Fusion, Nagaoka University of Technology.
2010: Associate professor; Department of Bioengineering, Nagaoka University of Technology.
2016: Professor; Department of Bioengineering, Nagaoka University of Technology.
In commemoration of receiving the ISS Research Awards 2016, "Space Station Top Results for Discoveries," the winners, Dr. Yasumitsu Sakamoto of Iwate Medical University's School of Pharmacy and Dr. Wataru Ogasawara of Nagaoka University of Technology, were invited to this second interview. They discuss a wide range of topics, such as when they were children and the future prospects for their research.
For your reference, you can find details about their research results presented in this interview article on the webpage below.
Press Release on Structural Studies on DAPBII(DPP7) in 2014Background to research
Congratulations on your winning the ISS Research Awards. First of all, I would like to ask you about the contents of your research in brief. You study proteins essential for the growth of microorganisms that cause periodontal disease. How did you start in the first place?
Dr. Sakamoto:
Thank you very much. I had a valuable experience at the award presentation ceremony held in San Diego. My first collaborative research with JAXA concerned a protein called DAP BII (DPP7), and Dr. Ogasawara is the one who discovered this protein.
Dr. Ogasawara:
That was more than 20 years ago when I was a first-year student in a master's program.

Dr. Sakamoto at the award presentation ceremony in 2016
How did you discover it?
Dr. Ogasawara:
Originally, my supervisor at the time (Dr. Yasushi Morikawa, professor emeritus, Nagaoka University of Technology) studied fungi, specializing in the study of enzymes that degrade cellulose. Cellulose is natural macromolecule that β-glucose polymerizes to form a single chain, and is a major component of plant cell walls. We cannot use intact cellulose because the molecule is too large. Therefore, cellulose must be decomposed into small molecules to enable its use. There are various decomposition mechanisms depending on the enzymes. For example, some enzymes cut a molecule at the middle of its molecular chain, while others sequentially digest a molecule from the end. Most enzymes that sequentially digest a molecule from the end cut "rings" in the chain one by one; however, there are some rare enzymes that cut the chain by removing two rings at a time.
On the other hand, protein is a nutrient source that consists of linearly connected amino acids. When we human beings eat protein, once it decomposes into amino acids, we use it as a nutrient. Enzyme that decomposes protein is called protease. Some proteases cut proteins at the middle of the chains, while others sequentially digest the molecule from the end. The proteases, which sequentially digest the molecule from the end, usually cut "rings" in the chain one by one in the same manner as cellulolytic (cellulose-degrading) enzymes. However, our laboratory already knew that there are enzymes that cut the chain two rings at a time. Therefore, we speculated that there should be bacteria having an enzyme that digests even protein from the end of the chain by removing two amino acids at a time, and actually tried looking for it. For your information, a chain consisting of multiple amino acids is called a peptide, and a molecule consisting of two amino acids connecting each other is called a dipeptide.
Our search was good hard work. Because every organism has a variety of proteolytic (protein-degrading) enzymes, and we could not distinguish whether we observed one reaction of an enzyme digesting two amino acids at a time, or whether we observed two reactions of the enzyme digesting the amino acids one by one, consequently removing two amino acids. No data were obtained for about two years since we started the search in the end.
We searched for bacteria in various places. We finally found that bacteria collected from the drainage of a tofu factory located in Nagaoka city, Niigata Prefecture, possess the enzyme of interest.
I see. It is likely that there are plenty of proteins to feed on microorganisms in the factories of milk, Calpis and tofu. Actually, is that easy to find special bacteria at such places?
Dr. Ogasawara:
The other day I had an occasion to speak with Professor Satoshi Omura, who was awarded the Nobel Prize in Physiology and Medicine in 2015. We talked about similar topics in that such special bacteria may be difficult to find, but must actually exist in various places at that time. I think it is true that it is easy to find in such places.
Then I examined the gene of the bacteria we found, but the gene was not homologous to the gene of any other bacterial species. Although we did not conduct a comprehensive investigation since we are not experts on bacteria, eventually it was confirmed as a new species by an expert. After that, I got an opportunity to work with Dr. Sakamoto regarding this newly discovered enzyme as just described.

Dr. Ogasawara explaining the enzymes that degrade cellulose
Dr. Sakamoto:
During the course of the research, this enzyme, which we named DAP BII (DPP7), was found to be important for the proliferation and growth of a group of bacteria, "non-fermental gram negative rods (NFGNR)," which live by using peptides and proteins as their nutrients. NFGNR include the causative bacteria of periodontal disease and hospital-acquired infection. Incidentally, periodontal disease is the most widespread infectious disease among human beings. When I started this study, I considered this enzyme to be both unusual and interesting. Then I also considered that if a substance could inhibit the function of DAP BII (DPP7), which is essential for growth of the pathogen, and other enzymes in the DPP family, the substance would become an antimicrobial agent. Thus, we also decided to develop antimicrobial agents.
So, you did not intend to make an antimicrobial agent from the start, but found that DAP BII (DPP7) was an excellent target of antimicrobial agents by chance.
Dr. Sakamoto:
Exactly. I also tried crystallization in parallel. However, the resolution did not improve easily although crystals were produced, and it did not go well for several years. At that time, I learned that there was a project to create high quality crystals in outer space. Then we decided to apply for the space experiments. That was around 2011.
Current research situation
Dr. Sakamoto:
There are several types of DPPs, each working differently. For example, DAP BII (DPP7) recognizes and cleaves hydrophobic or basic amino acids. On the other hand, DPP11 recognizes acidic amino acids. These enzymes are not found in humans, and are only found in microorganisms. In other words, if you can make medicines to suppress the function of these enzymes, it is harmless for humans and only suppresses the growth of bacteria.
There is another enzyme called DPP4, which specifically recognizes an amino acid called proline. Since humans have DPP4, I initially thought that it might be unsuitable as the target of antimicrobial development. However, a recent study showed that DPP4 in humans and microorganisms recognizes the same proline, but its way of recognition is different. DPP4 was successfully crystallized in space. If I can compare the binding mode of proline in detail, I feel that we could create drugs that only have an effect on DPP4 of microorganisms, but no effect on humans.
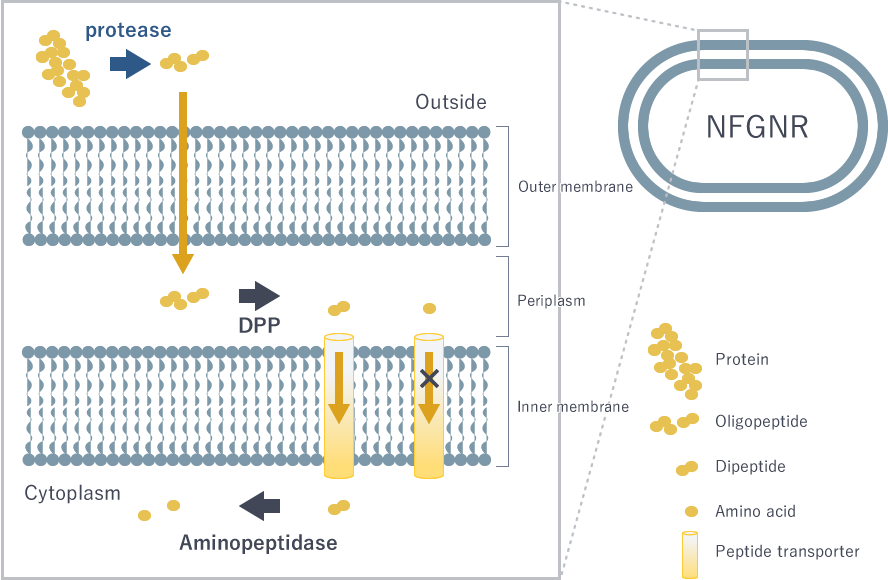
Expected mechanism of peptide uptake of NFGNR
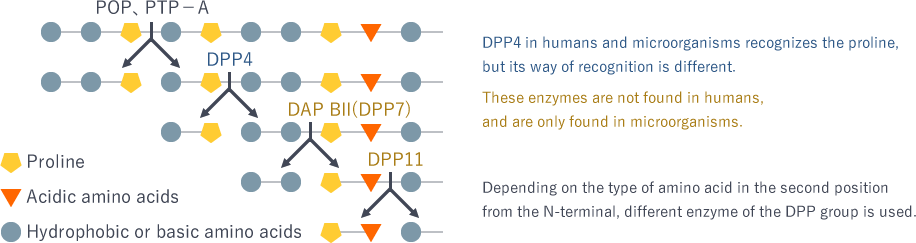
Substrate specificity of the DPP family
Proteins are broken down to oligopeptides consisting of about 10 amino acids outside the bacterial cell, and are transported to an area called periplasm in the bacteria. However, the peptide transporter, which transport peptides from the periplasm into the cytoplasm, only carries dipeptides and tripeptides, so it cannot be transported in the form of the oligopeptide. An enzyme that decomposes a peptide from the N terminal to two amino acids (dipeptide) is called DPP, and the amino acid to be recognized differs depending on the type of DPP. Suppressing the function of DPP makes it difficult for bacteria to proliferate.
In the first place, why does periodontal disease bacteria use dipeptides instead of amino acids?
Dr. Sakamoto:
I do not know. However, we know that absorption in the intestinal tract is faster for dipeptides than for amino acids in humans. Even if it is ingested as a form of dipeptide, it is eventually broken down into amino acids in the cell.
Dr. Ogasawara:
Fungi use cellulose as a nutrient source. Although amino acids and sugars are different, they are still taken in the form of disaccharides. But I do not know why. I can imagine various strategies, such as not wanting to be taken by other microbes or a strategy to coexist well with the surrounding environment. Now, to change the topic of conversation a bit, it seems that humans traditionally have an amino acid absorption system that makes it harder to develop diarrhea when amino acid is taken in the form of a dipeptide or tripeptide rather than a single amino acid.
In addition, I know a researcher at Waseda University who is good at binding amino acids to each other. According to him, there are sensors to recognize dipeptides on the tongue, and he found that the ability to taste salt becomes higher when we ingest salt and some dipeptides together. There are many people who are concerned about an excessive intake of salt, such as those with high blood pressure, but when you use such dipeptides, you can feel a taste with less salt. Besides that, we are finding that peptides have various functions. There are only 20 kinds of amino acids, but there are huge combinations between them.
Certainly, and these days I often hear that peptides were used as the precursors of pharmaceuticals and supplements.
Dr. Ogasawara:
I agree. I think peptides will get more and more attention from now on.
Research results so far and future developments
You have made many achievements so far. What was the most surprising thing about your previous research?
Dr. Ogasawara:
There are many proteolytic enzymes, but DPP is very different among them. Because it is so different, I thought that it was a mistake at the beginning. I was very surprised when it turned out that it was not a mistake. When the structure was further elucidated, the enzyme was found to take peptides in its "mouth," just like Pac-Man. Most proteases only have a upper part of the "face"; however, when I analyzed the structure of DPP, I was so surprised because it also has the lower part of the "face." After that, the structures during the eating of peptides, completely opening and closing their mouths, became clear. When we laid the data side-by-side, it looked like an animation. That was an "ooh and aah!" moment. It is common to explain by using the shape of the face to schematically show the function of the enzyme in textbooks and journals. I thought, "Oh, that was real!" (laughing)
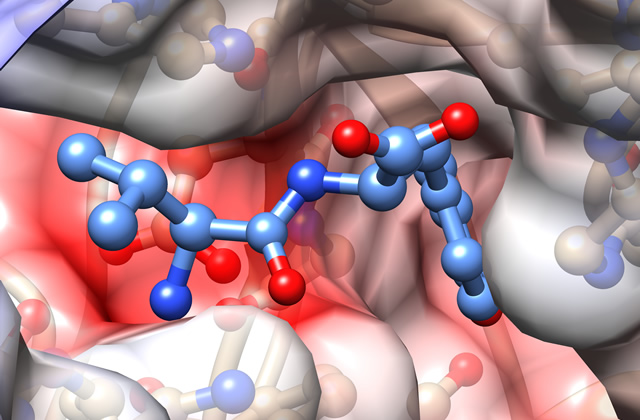
Binding of a peptide to DAP BII
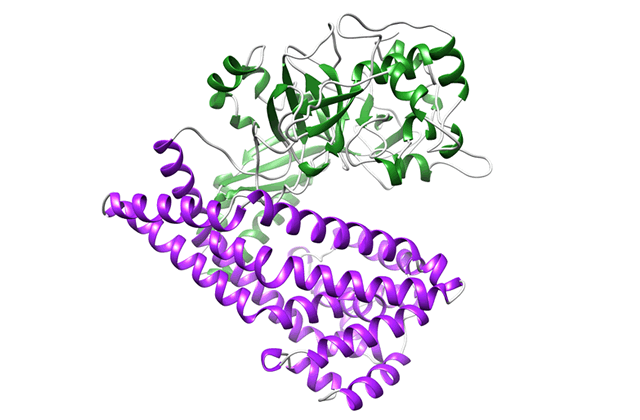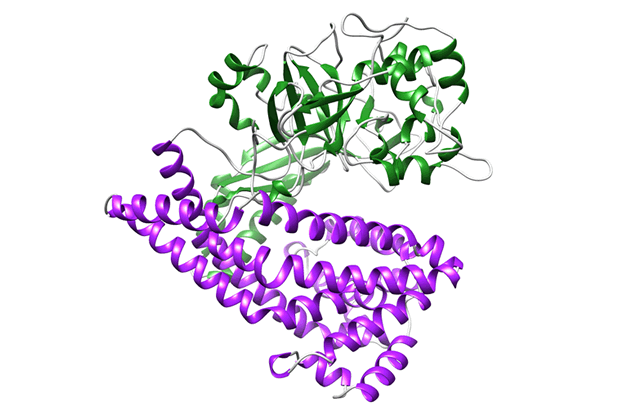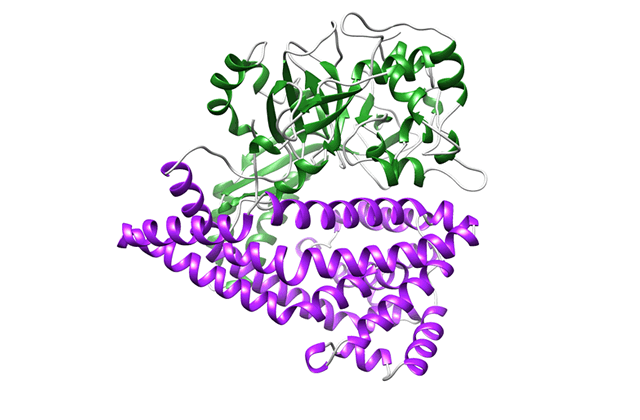
Comparison of the crystal structure of DAP BII
(opened form, semi-closed form and closed form)
Structural change of DAP BII predicted from crystal structure
Crystal structure of DPP11
Yes, I was surprised because it is just what I see in a textbook. What kind of prospects are you planning in the future?
Dr. Ogasawara:
For instance, regarding microorganisms, that I study, we know that bacteria exist not only in the mouth and intestines, but also in the heart. Now I'd like to find out, for example, where the bacteria are in the human body and what kind of influence the enzymes produced by them have on humans.
I have heard that one is prone to develop myocardial infarction if you do not brush your teeth.
Dr. Ogasawara:
That's right. Bacteria are known to exist in various places such as the heart, and the fact has also been published in major journals such as Nature. They are considered a cause of myocardial infarction and cerebral infarction, but this area of research area entails many unknown things. We are now only establishing the foundation of the research.
Do you mean that it is becoming known that there are many things you do not understand in this field?
Dr. Ogasawara:
Exactly. It means there are many things left to do.
Dr. Sakamoto:
First of all, in terms of application, I want to develop antibacterial drugs. Academically, I would like to elucidate the entire mechanism of peptide uptake. Dipeptides extracellularly produced by DPP are carried into cells by another protein, a peptide transporter.
It is known that a protease called trypsin and a peptide transporter form a complex in humans. Since DAPBII also has a structure similar to trypsin, DAPBII may also possibly interact with peptide transporters in microorganisms in the same manner as in humans.
Does that mean DPP may stick to the entrance of the peptide transporter so that the dipeptide can be taken up immediately into the cell?
Dr. Sakamoto:
That's right. It would be very interesting if that actually occurred. When these things are understood, it may be possible to make a medicine that not only simply inhibits the enzyme but also stops the delivery of dipeptides and inhibits complex formation.

Dr. Sakamoto explaining the future development
Space experiments
I would like to ask you about space experiments. Did you first become interested in the universe when you were a child?
Dr. Sakamoto:
I liked the rockets on display at the National Science Museum. And when I was in elementary school, I often read books about the universe from a collection of paperback scientific books called BLUE BACKS. There was a museum special issue, and I remember wanting to visit the Smithsonian before I die. Above all, the aerospace museum is particularly amazing.
When I was a child, I went to the Space Expo. It seems that I was more interested in machinery like the Space Shuttle than in gazing at the stars.
Dr. Ogasawara:
I had a small microscope and an astronomical telescope at home. I can never forget the time when I went to the mountains with my father after a typhoon had passed, and he showed me a starry sky that I had never seen before. I could see so many stars because it is dark in the country. Once I saw a glowing moving object in the sky that I thought might be a UFO and made some noise with my neighbors, although it was probably an artificial satellite. There are so many good images in the universe. In addition, I also read a monthly occult magazine called Mu. (laughing)
Did you ever think that you would join a space experiment?
Dr. Sakamoto:
Not at all. I could never imagine it. I thought, "Could I really do such a thing?" Initially, there was no idea about how the structural biology, which I study and space experiments are connected.
How did you learn about JAXA's space experiments?
Dr. Sakamoto:
Dr. Tanaka of Showa University (Dr. Nobutada Tanaka, associate professor, School of Pharmacy, Showa University) originally joined a space experiment. He knew that the resolution of my crystal did not improve, so he suggested that I use a space experiment. That was a cue.

Ms. Saori Roppongi (1st year student of a doctoral program) explaining the observation of crystals (back)
When I went to conferences and explaining our space experiment, many people told me that they knew about the experiment itself, but did not think that they could participate. Of course, you need to prepare proteins, but there may be special images in space experiments. I always feel keenly that there is a gap with the ease of reality entry. Therefore, I think we must work hard to communicate well.
Dr. Sakamoto:
Fortunately, we obtained good results since participating in the space experiment, although I burdened Dr. Ogasawara with the preparation of samples. (laughing)
AIST, which is our collaborative research institute, also kept asking us to analyze a variety of chemical compound candidates.
Dr. Ogasawara:
I think that it is a good thing because having a feeling of acceleration is important when things are going well. I also say to my students, "Oh, no, your samples may be too late."
Dr. Sakamoto:
Certainly, in fact, research has been accelerating thanks to the participation in space experiments.
In addition to the existing experiments conducted at 20°C, experiments at 4°C are also now being started, so you can use them as well, though it may cause you further strain.
Dr. Sakamoto:
Oh, is that so! Did you hear that, Dr. Ogasawara? Thank you for preparing the protein samples. (laughing)
Is there something like a decisive factor for success in space?
Dr. Sakamoto:
Well, I don't know. I also want to know. It is said that convection control is effective in improving the quality of crystals, but it is not actually visible. However, when comparing the experimental data as facts, the crystals obtained in space are always better than those on the ground .

Crystals of DAP IV obtained in space
Isn't it nice that the crystal returned from space had a high resolution after all?
Dr. Sakamoto:
Yes of course. I felt like, "Okay, come on!"
Did your impression change compared to when you first began participating in the space experiment?
Dr. Sakamoto:
It's been six years since I first participated in the space experiment. The intervals between space experiments have become shorter and shorter, and more opportunities for experiments are now available. It is extremely helpful because we can immediately apply the results of the previous experiment to the next one, and thus make use of that feedback in the next experiment. The preparation of samples and analysis of data have become harder, but we are very grateful to the JAXA staff that always gives us follow-up and support.
Can you recommend such participation to other researchers?
Dr. Sakamoto:
Yes of course. Although I would like to encourage other researchers, it may reduce my experimental opportunities. So, I don't recommend it in a very positive manner. (laughing)
School days
Now, I would like to ask you about your life before you became a researcher. How did you spend your school days, Dr. Ogasawara?
Dr. Ogasawara:
I am from Iwate. My results at school were so-so. My family operated a farm, so I learned many things by looking at things like a farmer's hardship and toughness, and others like management side by side.
After graduating from junior high school, I entered a technical college in Hachinohe (National Institute of Technology, Hachinohe College). Because the technical college is a five-year program, those who want to continue studying will transfer to the university. I studied chemistry at the college, but also liked biology. At that time, I met Dr. Yasuhiro Aoyama (professor of Nagaoka University of Technology) and he suggested that I enter the department of bioengineering, which was newly established at the Nagaoka University of Technology. In other words, I was a member of the inaugural class. Because we were the first students, there was not much stuff and no senior students, but we could try anything in such an environment. Dr. Tanaka of Showa University, a collaborator in this study, and I entered the program in the same year and were members of the inaugural class. Professor Senda, who is currently the director of the Structural Biology Research Center at KEK, also came to Nagaoka College of Technology instead of attending graduate school at the University of Tokyo. The department of bioengineering was resourceful on the part of both teachers and students, and I was well trained and had a good experience there.
After graduation, I enrolled in a doctoral program. By the way, both my brother and I promised that we get no money from our parents during our doctoral program, and we completed the program in that way. Today, some young students, especially those who transferred from a technical college, are not able to make up their minds about whether to go on to a doctoral program, because the background is different or concerns about money. But somehow, it will go well.
Even now I have opportunities to go to the college, and I often listen to students' concerns that they should have gone to a regular high school and then university, instead of a technical college. However, it is difficult to find what you want to do or have your teacher properly determine the suitable path for you during the three years in high school. In that sense, technical college offers a long time for you to find what you really want to do, and there is a lot of time to actually have experiences. So, I think these things are advantage of going to technical college.
When a professor at the technical college became a professor at Iwate University, about the time I received my doctoral degree, I was invited by the teacher and became an assistant professor at Iwate University. Then after a year and a half, I had an opportunity to study abroad in England. I think that it is not normally possible to study abroad immediately after assuming your post, but I thought it is important to find what you really want to do.
I majored in chemistry at college and then biology at university. At Iwate University, I was going to study in chemistry again. However, when I studied abroad the professor told me, "Please fuse genes and inorganic chemistry together." This meant to connect biology and chemistry. I thought it was a really tough request, but also thought that it was a chance.
For example, the spines of sea urchins in the ocean are structures made mainly of inorganic materials, sea urchins produce those needles. Because the functions of organisms are controlled by genes, I thought that research in the area of such topic would be the subject. By the way, the action of organisms to make inorganic minerals is called biomineralization. (Note 1) Back in those days, the study of biomineralization was advanced in the UK, so I thought about going to England.
(Note 1) Biomineralization is the process by which living organisms produce minerals (biological mineralization). The biomineralization by marine organisms such as shellfish and sea urchin are well known. They capture and concentrate various mineral ions dissolved in the ocean and produce shells, thorns and pearls.
Recently, the word biomineralization is being heard in many places. It is a very interesting field.
How did you spend your childhood, Dr. Sakamoto?
Dr. Sakamoto:
When I was in elementary school, I liked to go to museums. I lived in Saitama prefecture and I visited the National Science Museum, the Communications Museum, the Science Museum, and other museums alone.
I was a member of the school sciences club. I was the leader of the club in the third year. I signed up a summer school organized by Tsukuba Botanical Garden. I studied with botanists. I worked with Dr. Hiroaki Hatta (Emeritus researcher of the National Science Museum, PhD in Agricultural sciences) to survey the vegetation in Mt. Tsukuba and made plant specimens.
So, perhaps you were linked with Tsukuba since you were a child.
Dr. Sakamoto:
Indeed. When I was a high school student, I joined the brass band club because of a senior student there from the science department of my junior high school. I was too busy with the brass band and did not study at all, so my grades were literally at the bottom of my class.
(Everyone laughing)
Which instrument did you play in the brass band?
Dr. Sakamoto:
I played the horn. In the last year of high school, our band won a competition and advanced to a competition involving the entire Kanto region. The competition was held in September. Considering the remaining time until entrance examinations for universities in the winter, you can imagine what happed. After all, all boys in the band failed the exam.
So, you dedicated your high school life to a brass band?
Dr. Sakamoto:
You could say so. After preparing for the entrance examinations for another year, I entered the department of applied chemistry at Nihon University Junior College.
However, I originally liked biology, studied biology by myself, and took a transfer admission exam for Nagaoka University of Technology. A professor at Nihon University recommended that I take the exam. I didn't know until I transferred that most transferred students came from technical colleges. It seems I was the first student who transferred from a junior college.
After that, when I transferred to Nagaoka University of Technology, I found that Professor Mitsui's class was interesting. The content of his lectures focused on the structural analysis of proteins. I later belonged to his laboratory, which is the reason why I began research in this area.
Dr. Sakamoto's background seems to give courage to young people who are interested in research, but are not confident.
Dr. Sakamoto:
I certainly hope so. I currently serve as a member of a management and mentorship committee at my high school. Looking back on those days, I still wonder why I was invited. (laughing)
According to your story, you apparently encountered persons who changed your life many times. Is that true?
Dr. Sakamoto:
Yes, I believe encounters with certain people were important for me. I would not be what I am today without knowing the science club supervisor, professors at Nihon University, Dr. Yukio Mitsui at Nagaoka University of Technology, and Professor Takamasa Nonaka at Iwate Medical University. I feel that I am now conducting research like this as a result of thinking a great deal about such encounters.
Have you ever thought about getting a job at a company?
Dr. Sakamoto:
Graduate students of Nagaoka University of Technology were supposed to go on to a master's program, so I thought I went to graduate school. I did not think much about taking a job after that, and when Dr. Mitsui asked whether I would go on to a doctoral program when I was a first-year student of the master's program, I decided to enter the doctoral program.
Dr. Mitsui gave me opportunities for various experiences ever since I was an undergraduate student. When I was a first-year student of the master's program, I worked as a beam-line assistant at Photon Factory, the synchrotron radiation facility in Tsukuba. After that, I went to Dr. Natori's laboratory of the University of Tokyo for one year, and also went to Yamanouchi Pharmaceutical as an intern for half a year. My superior at that time was Dr. Sakashita (Dr. Hitoshi Sakashita, current general manager of the biomedical research division, AIST, who is also collaborator of us conducting drug discovery research. Dr. Mitsui gave me opportunities to meet a variety of interesting people.
Dr. Mitsui must be exactly your respected mentor.
Message to young people
I would appreciate if you could give any message to young people who are interested in research and reading this article.
Dr. Ogasawara:
I would say to young people to find out what you like as early as possible. However, specialization is necessary, but a wide range of interest is also important. A professor at the University of Cambridge also said, "It is important to have something you like, but it is not good when the interest is too narrow."
Exercise is also important. Students today are too serious, and they just do experiments if I leave them without saying anything. It is also necessary to do things with enjoyment as well.
However, in my case, what I dislike was rather clear. Of course, you can't avoid everything you don't want to do. So it is also important to take a pragmatic approach. When you conduct research, life is not all fun. You will experience many hardships, painful things, and even regrettable things. Even if you work at a company, a project may suddenly end for some reason. I recommend that you experience a variety of things while young so that you can go forward without becoming overwhelmed.
In that regard, I think that the system of Nagaoka University of Technology allows students to experience various things as compared with other universities. Actually, Nagaoka University of Technology became the best university chosen by company HR stuff in 2016. Nagaoka Technical College accepts many students who graduated from technical college. They are admitted as third year college students. These students have already trained in experiments by teachers of the technical college; therefore, at the university, they can smoothly begin experiments without any special guidance. Most university staff have yet to notice this point, but I think this is a big advantage of students from technical college. The field of research that you engage in at the university may be different from what you have already experienced, but actually previous experience leads to a major difference. Because students belong to a laboratory from the second semester of the third year at our university, students have the advantage of studying for three and a half years, including the master's program.
There is also an internship program in which students experience a company, a research institute like AIST, or overseas laboratories in the 4th year. Companies and laboratories train and educate students not only about research but also other aspects including safety in a strict manner, so students improve very much. For example, if parents get angry, children may not listen to what they say; however, if outsiders point out something, it works very well and quite differently(laughing). All students who participated in an internship program came back as a different person. In this way, it is important to accumulate experiences. There are many smart students, but it is difficult to cultivate tough persons. However, toughness is a very important quality for a researcher.
There are 51 technical colleges across the country from Asahikawa in the north to Okinawa in the south. Since the students gather from all over the country. They are diverse and interesting. There are not so many universities like them. So, this is one of its features. The environment of the technical college is also very good for research.
Dr. Sakamoto:
I also think that it is important to find dreams and what you want to do at first. You cannot study unless you feel you need it. You have to work hard to make your dreams come true. For example, if I did not submit my JAXA application, my research would not progress so much. However, one can notice this kind of thing only later on, so this is difficult. What I can say is that you should have a high regard for the people you met and what you experienced, and not miss out an opportunity, at the very least. And in my case, I enjoyed reading books very much.

Lab members of Nagaoka University of Technology and Iwate Medical University engaged in the research (at a laboratory at Iwate Medical University)
I see. If students experienced practical science and various things early, they may know which study they need, and it may also enhance their motivation.
Dr. Sakamoto:
I do not know many students (at Iwate Medical University) who have dreams, either. Most students want to become a pharmacist, but only few students want to become a researcher. Students in the Faculty of Pharmacy may not be much help because becoming a pharmacist is already their future goal at the admission stage. They are very realistic, though I do not know how they feel after assigned to a laboratory and experiencing research. I think that young people could be more adventurous.
Do you tell students that research is interesting?
Dr. Sakamoto:
I myself didn't say so but it would be nice if my students actually feel that research is interesting when they see me doing it, and actually try it and have fun by themselves.
Is there anything that students had better do before their graduation?
Dr. Sakamoto:
In my case, I got too involved in club activities and it eventually became a bit problem, but I learned cooperating with people and driving hard into something through it.
You cannot conduct research alone; in fact, I have been collaborating with various people, and feel that the abilities required for such collaboration, such as the capability for accommodation and collaboration as cultivated through the experience of club activities, are very useful now.
Dr. Ogasawara:
This may be happening throughout Japan, but all my students come to the laboratory for experiments, even on Saturday. I think you would be strained to the breaking point if you could not work happily. I guess they are all right. I am glad they are doing this because they want to do it. When and where to find what you want to do are different for each person. It may be better to find what you want to do earlier. But if you have not found it yet, please do not become discouraged.
I always have a burning passion as I grew up listening to songs by Yutaka Ozaki (a charismatic Japanese rock star). I often say that young people need to prepare themselves for when they find something they want to do, so that they are able to move into action immediately. Although it is not necessary to be as violent as to break the window of the school building as in an Ozaki's song. (laughing)
In addition, books are nice after all. They will never betray you, and will lead to an accumulation of knowledge in various areas.
At end of the interview
Today's guests have produced marked research results; on the other hand, their careers until becoming researchers were very unique. I hope their story offer them a fresh thinking and encouragement for who are interested in becoming researchers.
The students we chat with are all earnest and gentle. Their teachers must have a great influence.
We would like wish Dr. Sakamoto and Dr. Ogasawara even greater success in the future!
