
01

Why can high-quality crystals be grown in space? The answer is, because no convection occurs in space, but you may not be convinced by this explanation and remain woolly-minded. Let us explain a little more in detail, by referring to the previous research.
For protein crystallographers, a high-quality crystal is the one diffracting to a high resolution, and its diffraction data quality is high. This feature may be paraphrased by "single crystal" and "less lattice defects (crystal defects)." No one would demand a new definition of single crystal. However, a crystal look like a single crystal is not necessarily a single crystal. In fact, it might be a twin crystal or may contain micro crystals in it.
Several types of lattice defect affecting the crystal quality are known. The most critical one makes slight shifts of cell lattices over a certain range, resulting in the loss of periodicity of molecular alignment. This could be the main reason of crystals with high mosaicity, which is one of parameters to estimate diffraction data quality.
The two requirement for high-quality protein crystal, "single crystal" and "less lattice defects," can easily be achieved under microgravity. We describe step by step how high-quality crystals will grow under microgravity.
General outline of crystallization
Although many reagents are involved in an actual crystallization process, let's imagine only protein molecules are in the solution here. The initial phase of crystallization appears as shown in Fig. 1(a). With time, protein molecules meet each other and form a nucleus (Fig. 1(b)). The nucleus grows by incorporating the surrounding protein molecules. At this stage, the concentration of protein around the nucleus becomes lower than the bulk solution. (Fig. 1(c)). This lower protein concentration area is referred to as "depletion zone."
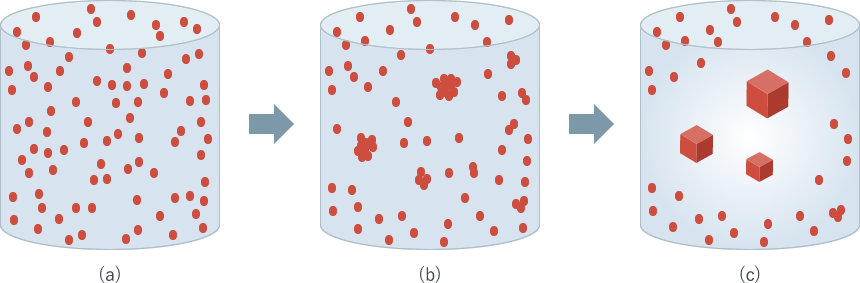
Fig. 1: The protein in the solution nucleates and grows into a crystal
Over time, the depletion zone moves upwards because its density is lower than the bulk solution. This phenomenon has been experimentally demonstrated (Pusey et al., J. Cryst. Growth 90, p.105 (1988)). The difference of concentration and density produce convection in a solution.
During crystallization, the solution with lower protein concentration moves upward and the solution with higher concentration comes close to the crystal instead. Thus, the protein concentration at the crystal surface is different depending on the crystal face, and changes with time. In other words, the protein concentration around a crystal is not constant in time and space.
How about crystallization under microgravity? Density difference convection occurs on earth. On the other hand, under the microgravity environment of the ISS, almost no density difference convection occurs in the solution. Without convection, protein molecules only move by diffusion. Thus, the depletion zone is maintained, and the growth of crystals is slow and stable.
Growth process of protein
The surface of a protein crystal observed with a laser confocal microscope looks like a terraced rice field (Fig. 2). The surface contains "steps." Each step height corresponds to a molecule in most cases. Protein molecules diffusing close to the crystal surface are captured at the steps into the crystalline layer. Thus, the steps change their positions and the crystal gradually increases its thickness.

Fig. 2: Surface of a lysozyme crystal observed with a laser confocal microscope
Fig. 3 shows the surface of a protein crystal observed with an atomic force microscope. As the concentration of protein in the solution rises, the surface of the crystal becomes rough. While the concentration is low in the solution, steps similar to those shown in Fig. 2 are observed (Fig. 3(a)(b)).
Fig. 3(b) shows the protein crystal surface growing in a higher protein concentration solution than in (a). With increased concentration, protein molecules meet each other on the crystal surface and form two-dimensional nuclei. These 2D islands capture other protein molecules on the steps on the end face to further grow in size and finally are combined with other 2D islands.
In cases of further increased concentration of the solution, several 2D islands are formed on top of a 2D island, forming a fine mountain-like shape (Fig. 3(c)). A slight disorder could be made among the unit lattices while such mountain-like structures are combined with each other. Voids can be created and impurities may be trapped there.
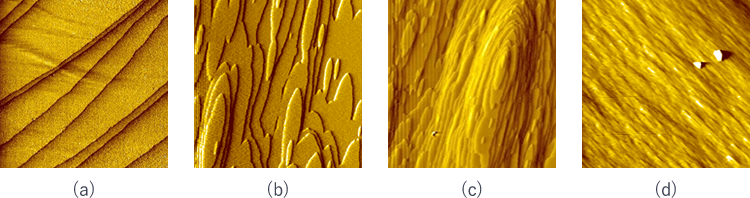
Fig. 3: Surface of a lysozyme crystal observed with an atomic force microscope
In a very high protein concentration, the crystal surface looks like as shown in Fig. 3(d). Protein molecules reach the surface one after another and steps are formed everywhere on the surface. Protein molecules must be incorporated in the correct orientation on the surface to form a high-quality crystal. However, the increased concentration of the protein accelerates the molecular interaction and disoriented molecules are settled on the crystalline layer. In other words, with lower protein concentration, molecules with disordered orientation are supposed to leave the crystal and return to the solution.
Both nucleation and crystal growth occur in a supersaturated solution. Nucleation requires higher level of supersaturation than crystal growth. This nature is more visible with protein. Therefore, growing high-quality protein crystals are often difficult.
In space, on the other hand, once crystal growth begins, the state of low protein concentration around the crystal (depletion zone) is maintained . Nucleation is suppressed and less number of crystal is grown, therefore less formations of clusters or multi-crystals. High-quality and defect-free crystals can be produced because they grow slowly and stably. Moreover, since no density difference convection occurs, impurities and aggregates are supposed to be less frequently brought onto the crystal surface than on Earth. Thus, such substances will not be captured by crystals and defect creation will be well controlled for better quality of crystals in space.
No sedimentation occurs in space
The specific gravity of a crystal is higher than that of the solution. Crystals sediment toward the bottom of the container on Earth. In fact, an observation showed that micro-crystals sedimented on the crystals which had already grown on the bottom of the container. Fig. 4 shows that micro-crystals that had been generated in the solution have sedimented on the mother crystal. Such micro-crystals will gradually be incorporated into the crystal in time. Such phenomena do not occur in space.
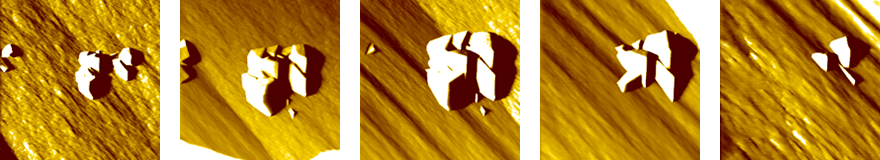
Fig. 4: Micro-crystals having sedimented on and being gradually incorporated into a crystal
We have described above the quality improvement mechanism of protein crystals under microgravity as commonly known at present. This mechanism has not completely been verified yet, but protein crystals having higher quality than on Earth have already been obtained through our previous space experiments. We will make further efforts to verify the mechanism and contribute to the global research on proteins, through high-quality protein crystal growth experiments in space.